BioMechanism of Use in Hair Loss
What is Laser Hair Therapy ?
Laser Therapy, also referred to as Low Level Laser Therapy (LLLT), cold laser therapy, photobiomodulation, biostimulation, and phototherapy, has been shown in thousands of peer-reviewed publications to increase cellular survival, proliferation and function. The laser light after absorbed by mitochondria in the cell produces the following actions
• Photo-Biomodulation and a stimulation effect
• Initiates protein synthesis
• Mobilizes calcium ions
• Enhancement of ATP production
• Significant improvement of blood microcirculation
• Allows greater nutrient acquisition
• Increases cellular respiration
• Induces activation of transcription factors via reactive oxygen species.
The question is no longer whether light has biological effects but rather how energy from therapeutic lasers and LEDs works at the cellular and organism levels and what the optimal light parameters are for different uses of these light sources.
Biphasic Dose Response
One important point that has been demonstrated by multiple studies in cell culture, animal models and in clinical studies is the concept of a biphasic dose response with the total delivered light energy density (fluence). The reason why the technique is termed Low-level is that there exists an optimal dose of light for any particular application, and dose lower than this optimum value, or more significantly, larger than the optimum value will have a diminished therapeutic outcome, or for high doses of light a negative outcome may result.
Delivering Methods
The methods for delivering the therapeutic light are diverse. The field is characterized by a variety of methodologies and uses of various light sources (lasers, LEDs) with different parameters (wavelength, output power, continuous-wave or pulsed operation modes, pulse parameters, polarization state etc). In 2002 MicroLight Corp received 510K FDA clearance for the ML 830-nm diode laser for treatment of carpal tunnel syndrome. There were several controlled trials reporting significant improvement in pain and some improvement in objective outcome measures. Since then several light sources have been approved as equivalent to an infra-red heating lamp for treating a wide-range of musculoskeletal disorders with no supporting clinical studies.
Hair Related Use of Laser
1. For Hair Loss
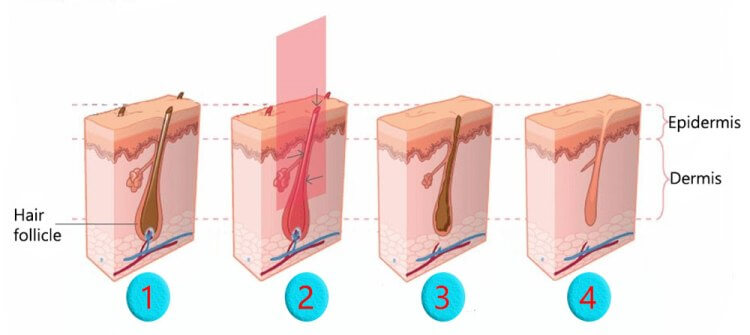
Laser is able to stimulate and preserve hair follicles in patients with androgenetic alopecia and other hair loss disorders. Laser has been used over the past few years in a number of laser devices (combs, caps, hairdryer-like) for treatment of genetic or acquired hair loss. The Laser energy addresses hair loss at the hair follicle cellular level, rejuvenating miniaturizing hair follicles in seven major ways:
• Photo-Biomodulation and a stimulation effect
• Initiates protein synthesis
• Mobilizes calcium ions within the hair follicle
• Mobilizes cellular stimulation within the dermal papilla
• Enhancement of ATP production in the cells
• Significant improvement of blood microcirculation
• Allows greater nutrient acquisition by follicular site
2. After Hair Transplant

Hair transplants can initially traumatize the scalp and can result in a temporary hair loss during the first 4 months (this is known as shock loss). Some patients may experience swelling in the transplanted area. The transplanted donor follicles can also experience difficulty adapting to their new environment. Clinical studies have demonstrated the following beneficial effects of laser when use in conjunction with hair transplant.
• minimizes hair shedding (shock loss)
• strengthen hair follicles after surgery with a much higher probability of survival
• reduce swelling, redness and inflammation post-surgery
Advantage 1 : Effect of Laser in Cell Energy Supply
Laser hair therapy stimulates the mitochondria in cells to increase the production of adenosine triphosphate (ATP). ATP is the form of energy used by hair cells to grow imto follicles. Abundant energy supply is critical when dealing with weakened and traumatized hair follicles.
Advantage 2 : Increase Success Rate after Hair Transplant
Laser hair therapy devices have been used by thousands of hair transplant centers all over the world (such as Bosley and HairClub). However handheld contraptions made with cheap Light Emitting Diodes (LEDs) are worthless when it comes to energizing the base of hair follicles. Technologically advanced device with the FDA-cleared is now available in our center for use after hair transplant. A 20 minutes of treatment is able to revive the mitochondria of hair cells. This can result in stronger hair follicles with a higher probability of surviving the operation. These extra amounts of “survivor” hair grafts will eventually grow into healthy, terminal hairs.
Other Clinical Uses of Laser
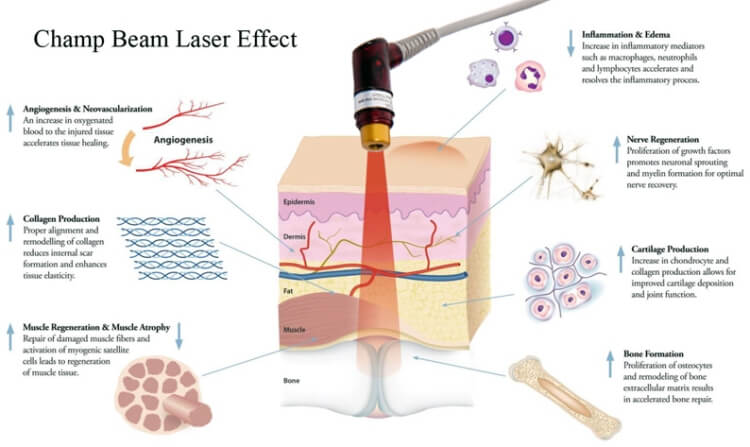
Promotes Wound Healing
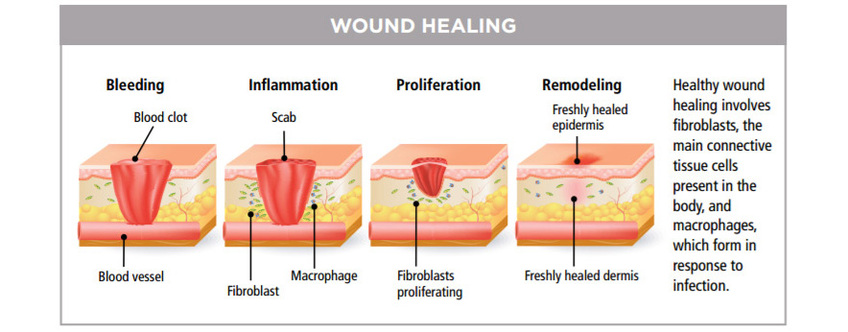
Since the first experiments in the 1960s with laser treatment for impaired wound healing, there has been much research-both lab-based and clinically-into the use of lasers for medical treatment. Later Nils Finsen pioneered the use of UV therapy for which he won the Nobel prize in 1904 [2]). The use of lasers and LEDs as light sources was the next step in the technological development of light therapy, which is now applied to many thousands of people worldwide each day.
The Lasers and LEDs are applied directly to the respective areas (e.g., wounds, sites of injuries) or to various points on the body (acupuncture points, muscle-trigger points). Controlled clinical trials have shown efficacy in treating stroke, stimulating wound healing, orthopedic conditions and relief of chronic inflammation. Preclinical studies have shown effectiveness in spinal cord injuries, peripheral nerve regeneration, heart attacks, degenerative brain diseases and traumatic brain injury.
Other Applications
• Sports-medicine - reduce swelling and hematoma, relieve pain, treat acute soft-tissue injuries
• Rehabilitation - improve mobility
• Dentistry - treat inflamed oral tissues and heal diverse ulcerations
• Dermatology - treat edema, non-healing ulcers, burns, and dermatitis
• Rheumatology - relieve pain and treat chronic inflammations and autoimmune diseases
• Veterinary Medicine - especially in racehorse-training centers
• Other specialists and general practitioners
Historical Development of LLLT
1967
Low Level Light therapy (LLLT) is one of the oldest therapeutic methods used by humans (historically as solar therapy by Egyptians). In 1967 a few years after the first working laser was invented, Endre Mester in Semmelweis University, Budapest, Hungary decided to test if laser radiation might cause cancer in mice. He shaved the hair off their backs, divided them into two groups and gave a laser treatment with a low powered ruby laser (694-nm) to one group. They did not get cancer and to his surprise the hair on the treated group grew back more quickly than the untreated group. This was the first demonstration of “laser biostimulation” to re-grow hair.
1989
It was suggested in 1989 that the mechanism of LLLT at the cellular level was based on the absorption of monochromatic visible and NIR radiation by components of the cellular respiratory chain [5]. Respiration occurs in subcellular organelles called mitochondria. The inner mitochondrial membrane contains 5 complexes of integral membrane proteins: NADH dehydrogenase (Complex I), succinate dehydrogenase (Complex II), cytochrome c reductase (Complex III), cytochrome c oxidase (Complex IV), ATP synthase (Complex V) and two freely-diffusible molecules ubiquinone and cytochrome c that shuttle electrons from one complex to the next. The respiratory chain accomplishes the stepwise transfer of electrons from NADH and FADH2 (produced in the citric acid or Krebs cycle) to oxygen molecules to form (with the aid of protons) water molecules harnessing the energy released by this transfer to the pumping of protons (H+) from the matrix to the intermembrane space. The gradient of protons formed across the inner membrane by this process of active transport forms a miniature battery. The protons can flow back down this gradient, reentering the matrix, only through another complex of integral proteins in the inner membrane, the ATP synthase complex.
1995
An analysis of five action spectra suggested that the primary photoacceptor for the red-NIR range in mammalian cells is cytochrome c oxidase [6] (Figure 2). It is remarkable that the action spectra that were analyzed had very close (within the confidence limits) peak positions in spite of the fact that these are seemingly different processes. The enzyme contains two iron centres, haem a and haem a3 (also referred to as cytochromes a and a3), and two copper centres, CuA and CuB [7]. Fully oxidized cytochrome c oxidase has both iron atoms in the Fe(III) oxidation state and both copper atoms in the Cu(II) oxidation state, while fully reduced cytochrome c oxidase has the iron in Fe(II) and copper in Cu(I) oxidation states. There are many intermediate mixed-valence forms of the enzyme and other coordinate ligands such as CO, CN, and formate can be involved. All the many individual oxidation states of the enzyme have different absorption spectra [8], thus probably accounting for slight differences in action spectra of LLLT that have been reported.
Recent Development
A recent paper from Karu’s group [9] gave the following wavelength ranges for four peaks in the LLLT action spectrum: 1) 613.5 – 623.5 nm, 2) 667.5 – 683.7 nm, 3) 750.7 – 772.3 nm, 4) 812.5 – 846.0 nm. Absorption of photons by molecules leads to electronically excited states and consequently can lead to acceleration of electron transfer reactions [10]. More electron transport necessarily leads to increased production of ATP [11]. Light induced increase in ATP synthesis and increased proton gradient leads to an increasing activity of the Na+/H+ and Ca2+/Na+ antiporters and of all the ATP driven carriers for ions, such as Na+/K+ ATPase and Ca2+ pumps. ATP is the substrate for adenyl cyclase, and therefore the ATP level controls the level of cAMP. Both Ca2+ and cAMP are very important second messengers. Ca2+ especially regulates almost every process in the human body (muscle contraction, blood coagulation, signal transfer in nerves, gene expression, etc.).

Our FDA Approved Laser Device - 1
For a number of years, there have been only two clinically proven non-surgical modalities by which the medical community and health care specialists have been able to treat hair loss. Minoxidil (Rogaine) and Finasteride (Propecia). For those who don't want to deal with chemicals or induced medical treatments, there is Laser Hair Therapy.
LaserCap (USA) Hong Kong Distributer

LaserCap re-energizes inactive hair follicles using Low-Level-Laser Therapy (LLLT), a safe, all-natural treatment, scientifically proven to promote hair regrowth in both men and women. The LaserCap is very easy to use. After charging the battery pack, insert LaserCap into your favorite hat, place the cap on your head, and turn it on for 30 minutes. Portable, hands-free, and discreet, LaserCap can be used in the comfort of your own home, at the gym, on your daily commute, anywhere and anytime that is convenient for you! Just wear the LaserCap for 30 minutes every other day to regrow and restore your natural hair.
FDA 510(k) Clearance
In order to protect people from false claims and dangerous devices, the United States requires stringent and rigorous testing and proof in the form of a 510(k) submission to the FDA before being granted clearance to be sold to the public. Testing includes clinical studies and laboratory data which must meet specific requirements after being reviewed by experts. LaserCap has been given this FDA 510(k) clearance regarding its SAFETY and EFFICACY as a Medical Device to grow hair and treat hair loss in both Men and Women.
Official Site
FDA Approved Laser Device - 2

Sunetic Laser (USA)
The Sunetics Laser Technology attacks hair loss at a cellular level using Bio-Stimulation to energize weakened follicles. Unlike oral finasteride or topical minoxidil, Sunetics laser hair therapy may be used by both men and women and is effective for hair regrowth. Our USA Imported Sunetics Model is known as the G Model. It has 107 diodes at 650nm and 5mW power. The open-hood design and laser pattern is designed to give 100% coverage to the treatment area. It was designed to treat Hair Loss and grow hair with the power of Laser Light. We provide free Sunetics Laser Therapy after our Hair Transplant to promote wound healing.
FDA 510(k) Clearance
Why is an FDA 510(k) Important? In the United States and abroad, thousands of devices are developed and placed on the market each year, claiming case studies and medical status. However, many of these claims are backed up with little more than words. In order to protect people from false claims and dangerous devices, the United States requires stringent and rigorous testing and proof in the form of a 510(k) submission to the FDA before being granted clearance to be sold to the public.
The purpose of this submission is to prove the SAFETY and EFFICACY of a Medical Device. This testing includes clinical studies and laboratory data provided by the applicant. This data must meet specific requirements and is then reviewed by experts. Only after gaining approval by these experts is an FDA 510(k) cleared status given to a device. Sunetics International brings the first Clinical Laser Unit FDA 510(k) cleared to grow hair and treat hair loss in both Men and Women. Please refer to Sunetics Site for more details.
Scientific Study - Laser for Hair Growth
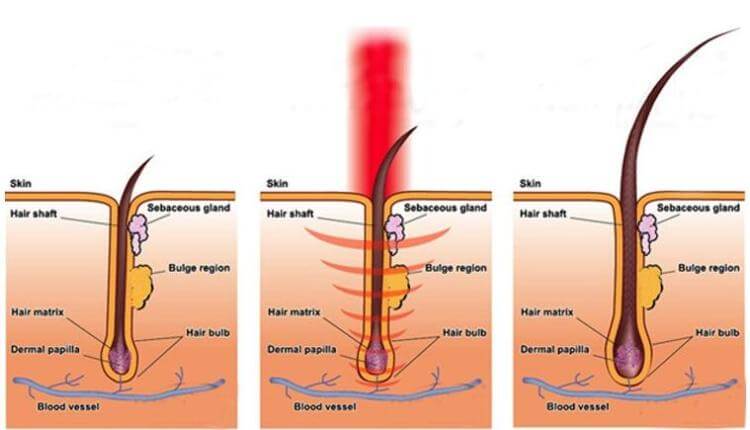
Dr Hamblin 2009
Since the first pioneering publication of Mester [1] reported stimulation of hair growth in mice, there have been virtually no follow-up studies on LLLT stimulation for hair growth in animal models. Mester’s study involved delivering 1 J of pulsed light (1 millisecond pulse duration) into a 1 cm2 spot from a ruby laser at 694-nm to the depilated abdominal area of black C57 and white Balb/c mice every week for up to 11 weeks. Before each successive treatment the skin was again depilated. Increased hair growth in the irradiated spot growth was observed in all black animals between the 5th and 7th treatment. This reaction continued to the 9th treatment and it was characteristic of the hair growth intensity that in places that were completely bare at the time of the respective irradiation, hair growth as dense as on other body parts was observed only 4 – 6 days after the irradiation. On the other hand, it was found after the 9th irradiation that hair growth stopped in the irradiated locations only. Instead, a peripheral, ringshaped hair growth was observed around the irradiated area. This ring-shaped hair growth first appeared in the animal on which the central growth stimulation was first observed. The peripheral growth appeared in all treated black mice between the 7th and 9th irradiation with the intensity varying from mouse to mouse. In white mice no effect on hair growth was detected up to the 8th irradiation. The central growth described for black mice only began to form after the 8th irradiation. Further irradiation caused the hair growth just described in some of the mice, but the peripheral hair growth characteristic of the 2nd phase was already appearing in some as well. The hair growth of the control animals developed as follows: The depilated skin grew hair slowly and diffusely. However, on half of the control animals (both among black and white mice), no further hair growth whatsoever was observed. At the same time, a diffuse hair growth appeared on some animals, but in other animals an uncharacteristic, sometimes diagonal strip appeared.
Despite the fact that LLLT devices are widely marketed and used for hair regrowth, there have been only a few literature reports containing some observations of LLLT-induced hair growth in patients, and amelioration or treatment of any type of alopecia. A Japanese group reported [49] on the use of Super Lizer (a linear polarized light source providing 1.8W of 600 – 1600-nm light) to treat alopecia areata. Three minute sessions every one or two weeks produced significant hair growth compared to non-treated lesions in 47% of patients. A Spanish group has reported [50, 51] on the use of HeNe laser for both alopecia androgenic and areata. A report from Finland [52] compared three different light sources used for male-pattern baldness (HeNe laser, InGaAl diode laser at 670-nm and non-coherent 635-nm LED and measured blood flow in the scalp.
Recent work has uncovered some biological mechanisms involved in the regulation of hair growth that could be good candidates to explain the stimulating effects of LLLT. Peters et al [53] found that nerve growth factor (NGF) promotes proliferation via its high affinity receptor (TrkA). and identified NGF and p75 as important hair growth terminators. By rtPCR we found, that NGF/proNGF mRNA levels peak during early anagen in murine back skin while NGF/proNGF protein levels peak during catagen, indicating high turnover in early anagen and protein accumulation in catagen. By immunohistochemistry, NGF and TrkA were found in the proliferating compartments of the epidermis and hair follicle throughout the cycle. Commercial 7S NGF, which contains both NGF and proNGF, promotes anagen development in organ-cultured early anagen mouse skin, while it promotes catagen development in late anagen skin. Therefore the data suggest an anagen-promoting/-supporting role for NGF/TrkA.
Another report from this group [54] studied the expression and function of p75 neurotrophin receptor (p75NTR), which is implicated in apoptosis control in spontaneous catagen development in murine skin. They found that p75NTR alone was strongly expressed in TUNEL+/Bcl2- keratinocytes of the regressing outer root sheath, but both p75NTR and TrkB and/or TrkC were expressed by the nonregressing TUNEL- /Bcl2+ secondary hair germ keratinocytes. There was significant catagen retardation in p75NTR knockout mice as compared to wild-type controls. Instead, transgenic mice over expressing NGF (promoter: K14) showed substantial acceleration of catagen.Schwartz et al [55] reported in 2002 that helium/neon laser irradiation (3J/cm2) augmented the level of NGF mRNA fivefold and increased NGF release to the medium of myotubes cultured in vitro. This correlated with a transient elevation of intracellular calcium in the myotubes. Yu and coworkers found a significant increase in nerve growth factor release from cultured human keratinocytes. [27]. Therefore it is postulated that LLLT may influence hair regrowth via the NGF/ p75NTR signaling system.
Zcharia and colleagues [56] identified the endoglycosidase, heparanase, as an Important regulator of murine hair growth. Degradation of the extracellular matrix barrier formed by heparan sulfate by heparanase enables cell movement through extracellular barriers and releases growth factors from extracellular matrix depots, making them bioavailable. This allows follicular stem cell progeny migration and reconstitution of the lower part of the follicle, which is a prerequisite for hair shaft formation. Heparanase contributed to the ability of the bulge-derived keratinocytes to migrate through the extracellular matrix barrier in vitro. In heparanase-overexpressing transgenic mice, increased levels of heparanase enhanced active hair growth and enabled faster hair recovery after chemotherapy-induced alopecia. Thymosin beta4 (TB4) is a 43-amino acid polypeptide that is an important mediator of cell migration and differentiation, also promotes angiogenesis and wound healing [57]. Philp et al [58] reported that TB4 stimulated hair growth in normal rats and mice. A specific subset of hair follicular keratinocytes in mouse skin expressed TB4 in a highly coordinated manner during the hair growth cycle. These keratinocytes originated in the hair follicle bulge region, a niche for skin stem cells. Rat vibrissa follicle clonogenic keratinocytes, closely related, if not identical, to the bulge-residing stem cells, were isolated and their migration and differentiation increased in the presence of nanomolar concentrations of TB4. Expression and secretion of the extracellular matrix degrading enzyme matrix metalloproteinase-2 were increased by TB4. Thus, TB4 accelerated hair growth, in part, due to its effect on critical events in the active phase of the hair follicle cycle, including promoting the migration of stem cells and their immediate progeny to the base of the follicle, differentiation, and extracellular matrix remodeling.
A recent report [59] identified the transforming growth factor-beta family member activin is a potent regulator of skin morphogenesis, repair and hair growth. Mice overexpressing the secreted activin antagonist follistatin, however, have the reduced hair growth. Mice expressing a dominant-negative activin receptor IB mutant (dnActRIB) in keratinocytes had unaltered architecture of adult skin, but delays were observed in postnatal pelage hair follicle morphogenesis and in the first catagen-telogen transformation of hair follicles.As yet there are no reports of LLLT affecting heparanase, TB4, or activin expression levels in tissue culture or in mouse skin, but these molecules are good candidates for further study to explain the hair growth-induction by LLLT.
Medical References
More than 1,000 publications have reported that laser or low energy lasers can effectively increase cell survival, proliferation and function. Clinical controlled trials have shown that lasers can stimulate and preserve hair follicles affected by androgenetic alopecia and other alopecia.
[1] E. Mester, B. Szende and P. Gartner, The effect of laser beams on the growth of hair in mice, Radiobiol Radiother (Berl) 9 (1968) 621-6.
[2] R. Roelandts, The history of phototherapy: something new under the sun?, J Am Acad Dermatol 46 (2002) 926-30.
[3] A.N. Pereira, P. Eduardo Cde, E. Matson and M.M. Marques, Effect of low-power laser irradiation on cell growth and procollagen synthesis of cultured fibroblasts, Lasers Surg Med 31 (2002) 263-7.
[4] J.S. Kana, G. Hutschenreiter, D. Haina and W. Waidelich, Effect of low-power density laser radiation on healing of open skin wounds in rats, Arch Surg 116 (1981) 293-6.
[5] A.P. Sommer, A.L. Pinheiro, A.R. Mester, R.P. Franke and H.T. Whelan, Biostimulatory windows in low-intensity laser activation: lasers, scanners, and NASA’s light-emitting diode array system, J Clin Laser Med Surg 19 (2001) 29-33.
[6] J.C. Sutherland, Biological effects of polychromatic light, Photochem Photobiol 76 (2002) 164-70.
[7] T. Karu, Laser biostimulation: a photobiological phenomenon, J Photochem Photobiol B 3 (1989) 638-40.
[8] T.I. Karu and N.I. Afanas’eva, Cytochrome c oxidase as the primary photoacceptor upon laser exposure of cultured cells to visible and near IR-range light, Dokl Akad Nauk 342 (1995) 693-5.
[9] R.A. Capaldi, F. Malatesta and V.M. Darley-Usmar, Structure of cytochrome c oxidase, Biochim Biophys Acta 726 (1983) 135-48.
[10] I. Szundi, G.L. Liao and O. Einarsdottir, Near-infrared time-resolved optical absorption studies of the reaction of fully reduced cytochrome c oxidase with dioxygen, Biochemistry 40 (2001) 2332-9.
[11] T.I. Karu and S.F. Kolyakov, Exact action spectra for cellular responses relevant to phototherapy, Photomed Laser Surg 23 (2005) 355-61.
[12] D. Pastore, M. Greco and S. Passarella, Specific helium-neon laser sensitivity of the purified cytochrome c oxidase, Int J Radiat Biol 76 (2000) 863-70.
[13] V.G. Artyukhov, O.V. Basharina, A.A. Pantak and L.S. Sveklo, Effect of helium-neon laser on activity and optical properties of catalase, Bull Exp Biol Med 129 (2000) 537-40.
[14] W. Yu, J.O. Naim, M. McGowan, K. Ippolito and R.J. Lanzafame, Photomodulation of oxidative metabolism and electron chain enzymes in rat liver mitochondria, Photochem Photobiol 66 (1997) 866-71.
[15] S. Passarella, He-Ne laser irradiation of isolated mitochondria, J Photochem Photobiol B 3 (1989) 642-3.
[16] S.J. Ehrreich and R.F. Furchgott, Relaxation of mammalian smooth muscles by visible and ultraviolet radiation, Nature 218 (1968) 682-4.
[17] H. Chaudhry, M. Lynch, K. Schomacker, R. Birngruber, K. Gregory and I. Kochevar, Relaxation of vascular smooth muscle induced by low-power laser radiation, Photochem Photobiol 58 (1993) 661-9.
[18] R. Mittermayr, A. Osipov, C. Piskernik, S. Haindl, P. Dungel, C. Weber, Y.A. Vladimirov, H. Redl and A.V. Kozlov, Blue laser light increases perfusion of a skin flap via release of nitric oxide from hemoglobin, Mol Med 13 (2007) 22-9.
[19] L. Vladimirov, A., G.I. Klebanov, G.G. Borisenko and A.N. Osipov, Molecular and cellular mechanisms of the low intensity laser radiation effect, Biofizika 49 (2004) 339-50.
[20] Y.A. Vladimirov, A.N. Osipov and G.I. Klebanov, Photobiological principles of therapeutic applications of laser radiation, Biochemistry (Mosc) 69 (2004) 81-90.
[21] G.G. Borisenko, A.N. Osipov, K.D. Kazarinov and A. Vladimirov Yu, Photochemical reactions of nitrosyl hemoglobin during exposure to low-power laser irradiation, Biochemistry (Mosc) 62 (1997) 661-6.
[22] B. Beltran, A. Mathur, M.R. Duchen, J.D. Erusalimsky and S. Moncada, The effect of nitric oxide on cell respiration: A key to understanding its role in cell survival or death, Proc Natl Acad Sci U S A 97 (2000) 14602-7.
[23] G.C. Brown, Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase, Biochim Biophys Acta 1504 (2001) 46-57.
[24] N. Lane, Cell biology: power games, Nature 443 (2006) 901-3.
[25] G.C. Brown and V. Borutaite, Nitric oxide inhibition of mitochondrial respiration and its role in cell death, Free Radic Biol Med 33 (2002) 1440-50.
[26] P. Ghafourifar and E. Cadenas, Mitochondrial nitric oxide synthase, Trends Pharmacol Sci 26 (2005) 190-5.
[27] E. Clementi, G.C. Brown, N. Foxwell and S. Moncada, On the mechanism by which vascular endothelial cells regulate their oxygen consumption, Proc Natl Acad Sci U S A 96 (1999) 1559-62.
[28] F. Antunes, A. Boveris and E. Cadenas, On the mechanism and biology of cytochrome oxidase inhibition by nitric oxide, Proc Natl Acad Sci U S A 101 (2004) 16774-9.
[29] T.I. Karu, L.V. Pyatibrat and N.I. Afanasyeva, Cellular effects of low power laser therapy can be mediated by nitric oxide, Lasers Surg Med 36 (2005) 307-14.
[30] V. Borutaite, A. Budriunaite and G.C. Brown, Reversal of nitric oxide-, peroxynitrite- and S-nitrosothiol-induced inhibition of mitochondrial respiration or complex I activity by light and thiols, Biochim Biophys Acta 1459 (2000) 405-12.
[31] G.A. Guzzardella, M. Fini, P. Torricelli, G. Giavaresi and R. Giardino, Laser stimulation on bone defect healing: an in vitro study, Lasers Med Sci 17 (2002) 216-20.
[32] H. Tuby, L. Maltz and U. Oron, Modulations of VEGF and iNOS in the rat heart by low level laser therapy are associated with cardioprotection and enhanced angiogenesis, Lasers Surg Med 38 (2006) 682-8.
[33] Y. Moriyama, E.H. Moriyama, K. Blackmore, M.K. Akens and L. Lilge, In vivo study of the inflammatory modulating effects of low-level laser therapy on iNOS expression using bioluminescence imaging, Photochem Photobiol 81 (2005) 1351-5.
[34] M.C. Leung, S.C. Lo, F.K. Siu and K.F. So, Treatment of experimentally induced transient cerebral ischemia with low energy laser inhibits nitric oxide synthase activity and up-regulates the expression of transforming growth factor-beta 1, Lasers Surg Med 31 (2002) 283-8.
[35] H. Friedmann, R. Lubart, I. Laulicht and S. Rochkind, A possible explanation of laser-induced stimulation and damage of cell cultures, J Photochem Photobiol B 11 (1991) 87-91.
[36] M. Eichler, R. Lavi, A. Shainberg and R. Lubart, Flavins are source of visible-light-induced free radical formation in cells, Lasers Surg Med 37 (2005) 314-9.
[37] K. Plaetzer, T. Kiesslich, B. Krammer and P. Hammerl, Characterization of the cell death modes and the associated changes in cellular energy supply in response to AlPcS4-PDT, Photochem Photobiol Sci 1 (2002) 172-7.
[38] R. Lubart, M. Eichler, R. Lavi, H. Friedman and A. Shainberg, Low-energy laser irradiation promotes cellular redox activity, Photomed Laser Surg 23 (2005) 3-9.
[39] R. Duan, T.C. Liu, Y. Li, H. Guo and L.B. Yao, Signal transduction pathways involved in low intensity He-Ne laser-induced respiratory burst in bovine neutrophils: a potential mechanism of low intensity laser biostimulation, Lasers Surg Med 29 (2001) 174-8.
[40] F.Q. Schafer and G.R. Buettner, Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple, Free Radic Biol Med 30 (2001) 1191-212.
[41] H. Liu, R. Colavitti, Rovira, II and T. Finkel, Redox-dependent transcriptional regulation, Circ Res 97 (2005) 967-74.
[42] M. Yang, N.B. Nazhat, X. Jiang, S.M. Kelsey, D.R. Blake, A.C. Newland and C.J. Morris, Adriamycin stimulates proliferation of human lymphoblastic leukaemic cells via a mechanism of hydrogen peroxide (H2O2) production, Br J Haematol 95 (1996) 339-44.
[43] W.G. Kirlin, J. Cai, S.A. Thompson, D. Diaz, T.J. Kavanagh and D.P. Jones, Glutathione redox potential in response to differentiation and enzyme inducers, Free Radic Biol Med 27 (1999) 1208-18.
[44] S. Alaluf, H. Muir-Howie, H.L. Hu, A. Evans and M.R. Green, Atmospheric oxygen accelerates the induction of a post-mitotic phenotype in human dermal fibroblasts: the key protective role of glutathione, Differentiation 66 (2000) 147-55.
[45] T. Karu, Primary and secondary mechanisms of action of visible to near-IR radiation on cells, J Photochem Photobiol B 49 (1999) 1-17.
[46] O. Tiphlova and T. Karu, Action of low-intensity laser radiation on Escherichia coli, Crit Rev Biomed Eng 18 (1991) 387-412.
[47] T.I. Karu, L.V. Pyatibrat, G.S. Kalendo and R.O. Esenaliev, Effects of monochromatic low-intensity light and laser irradiation on adhesion of HeLa cells in vitro, Lasers Surg Med 18 (1996) 171-7.
[48] P. Moore, T.D. Ridgway, R.G. Higbee, E.W. Howard and M.D. Lucroy, Effect of wavelength on low-intensity laser irradiation-stimulated cell proliferation in vitro, Lasers Surg Med 36 (2005) 8-12.
[49] D. Hawkins and H. Abrahamse, Biological effects of helium-neon laser irradiation on normal and wounded human skin fibroblasts, Photomed Laser Surg 23 (2005) 251-9.
[50] H.S. Yu, C.S. Wu, C.L. Yu, Y.H. Kao and M.H. Chiou, Helium-neon laser irradiation stimulates migration and proliferation in melanocytes and induces repigmentation in segmental-type vitiligo, J Invest Dermatol 120 (2003) 56-64.
[51] S. Young, P. Bolton, M. Dyson, W. Harvey and C. Diamantopoulos, Macrophage responsiveness to light therapy, Lasers Surg Med 9 (1989) 497-505.
[52] Y. Fujimaki, T. Shimoyama, Q. Liu, T. Umeda, S. Nakaji and K. Sugawara, Low-level laser irradiation attenuates production of reactive oxygen species by human neutrophils, J Clin Laser Med Surg 21 (2003) 165-70.
[53] Y.S. Chen, S.F. Hsu, C.W. Chiu, J.G. Lin, C.T. Chen and C.H. Yao, Effect of low-power pulsed laser on peripheral nerve regeneration in rats, Microsurgery 25 (2005) 83-9.
[54] M. Miloro, L.E. Halkias, S. Mallery, S. Travers and R.G. Rashid, Low-level laser effect on neural regeneration in Gore-Tex tubes, Oral Surg Oral Med Oral Pathol Oral Radiol Endod 93 (2002) 27-34.
[55] P. Balaban, R. Esenaliev, T. Karu, E. Kutomkina, V. Letokhov, A. Oraevsky and N. Ovcharenko, He-Ne laser irradiation of single identified neurons, Lasers Surg Med 12 (1992) 329-37.
[56] K.R. Byrnes, R.W. Waynant, I.K. Ilev, X. Wu, L. Barna, K. Smith, R. Heckert, H. Gerst and J.J. Anders, Light promotes regeneration and functional recovery and alters the immune response after spinal cord injury, Lasers Surg Med 36 (2005) 171-85.
[57] S.O. el Sayed and M. Dyson, Effect of laser pulse repetition rate and pulse duration on mast cell number and degranulation, Lasers Surg Med 19 (1996) 433-7.
[58] R.A. Lopes-Martins, R. Albertini, P.S. Martins, J.M. Bjordal and H.C. Faria Neto, Spontaneous effects of low-level laser therapy (650 nm) in acute inflammatory mouse pleurisy induced by Carrageenan, Photomed Laser Surg 23 (2005) 377-81.
[59] A.D. Agaiby, L.R. Ghali, R. Wilson and M. Dyson, Laser modulation of angiogenic factor production by T-lymphocytes, Lasers Surg Med 26 (2000) 357-63.
[60] S. Passarella, E. Casamassima, S. Molinari, D. Pastore, E. Quagliariello, I.M. Catalano and A. Cingolani, Increase of proton electrochemical potential and ATP synthesis in rat liver mitochondria irradiated in vitro by helium-neon laser, FEBS Lett 175 (1984) 95-9.
[61] M. Greco, G. Guida, E. Perlino, E. Marra and E. Quagliariello, Increase in RNA and protein synthesis by mitochondria irradiated with helium-neon laser, Biochem Biophys Res Commun 163 (1989) 1428-34.
[62] D. Pastore, M. Greco, V.A. Petragallo and S. Passarella, Increase in H+/e- ratio of the cytochrome c oxidase reaction in mitochondria irradiated with helium-neon laser, Biochem Mol Biol Int 34 (1994) 817-26.
[63] Y. Zhang, S. Song, C.C. Fong, C.H. Tsang, Z. Yang and M. Yang, cDNA microarray analysis of gene expression profiles in human fibroblast cells irradiated with red light, J Invest Dermatol 120 (2003) 849-57.
[64] R.F. Lyons, R.P. Abergel, R.A. White, R.M. Dwyer, J.C. Castel and J. Uitto, Biostimulation of wound healing in vivo by a helium-neon laser, Ann Plast Surg 18 (1987) 47-50.
[65] H.S. Yu, K.L. Chang, C.L. Yu, J.W. Chen and G.S. Chen, Low-energy helium-neon laser irradiation stimulates interleukin-1 alpha and interleukin-8 release from cultured human keratinocytes, J Invest Dermatol 107 (1996) 593-6.
[66] V.K. Poon, L. Huang and A. Burd, Biostimulation of dermal fibroblast by sublethal Q-switched Nd:YAG 532 nm laser: collagen remodeling and pigmentation, J Photochem Photobiol B 81 (2005) 1-8.
[67] N. Kipshidze, V. Nikolaychik, M.H. Keelan, L.R. Shankar, A. Khanna, R. Kornowski, M. Leon and J. Moses, Low-power helium: neon laser irradiation enhances production of vascular endothelial growth factor and promotes growth of endothelial cells in vitro, Lasers Surg Med 28 (2001) 355-64.
[68] A. Khanna, L.R. Shankar, M.H. Keelan, R. Kornowski, M. Leon, J. Moses and N. Kipshidze, Augmentation of the expression of proangiogenic genes in cardiomyocytes with low dose laser irradiation in vitro, Cardiovasc Radiat Med 1 (1999) 265-9.
[69] A.R. Medrado, L.S. Pugliese, S.R. Reis and Z.A. Andrade, Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts, Lasers Surg Med 32 (2003) 239-44.
[70] E.J. Neiburger, Rapid healing of gingival incisions by the helium-neon diode laser, J Mass Dent Soc 48 (1999) 8-13, 40.
[71] J.T. Eells, M.M. Henry, P. Summerfelt, M.T. Wong-Riley, E.V. Buchmann, M. Kane, N.T. Whelan and H.T. Whelan, Therapeutic photobiomodulation for methanol-induced retinal toxicity, Proc Natl Acad Sci U S A 100 (2003) 3439-44.
[72] M.T. Wong-Riley, H.L. Liang, J.T. Eells, B. Chance, M.M. Henry, E. Buchmann, M. Kane and H.T. Whelan, Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase, J Biol Chem 280 (2005) 4761-71.
[73] D. Gigo-Benato, S. Geuna and S. Rochkind, Phototherapy for enhancing peripheral nerve repair: a review of the literature, Muscle Nerve 31 (2005) 694-701.
[74] J.J. Anders, S. Geuna and S. Rochkind, Phototherapy promotes regeneration and functional recovery of injured peripheral nerve, Neurol Res 26 (2004) 233-9.
[75] J.J. Anders, R.C. Borke, S.K. Woolery and W.P. Van de Merwe, Low power laser irradiation alters the rate of regeneration of the rat facial nerve, Lasers Surg Med 13 (1993) 72-82.
[76] K. Branco and M.A. Naeser, Carpal tunnel syndrome: clinical outcome after low-level laser acupuncture, microamps transcutaneous electrical nerve stimulation, and other alternative therapies–an open protocol study, J Altern Complement Med 5 (1999) 5-26.
[77] J. Irvine, S.L. Chong, N. Amirjani and K.M. Chan, Double-blind randomized controlled trial of low-level laser therapy in carpal tunnel syndrome, Muscle Nerve 30 (2004) 182-7.
[78] M.I. Weintraub, Noninvasive laser neurolysis in carpal tunnel syndrome, Muscle Nerve 20 (1997) 1029-31.
[79] Y. Ueda and N. Shimizu, Pulse irradiation of low-power laser stimulates bone nodule formation, J Oral Sci 43 (2001) 55-60.
[80] Y. Ueda and N. Shimizu, Effects of pulse frequency of low-level laser therapy (LLLT) on bone nodule formation in rat calvarial cells, J Clin Laser Med Surg 21 (2003) 271-7.
[81] M.S. Ribeiro, F. Da Silva Dde, C.E. De Araujo, S.F. De Oliveira, C.M. Pelegrini, T.M. Zorn and D.M. Zezell, Effects of low-intensity polarized visible laser radiation on skin burns: a light microscopy study, J Clin Laser Med Surg 22 (2004) 59-66.
[82] T. Moshkovska and J. Mayberry, It is time to test low level laser therapy in Great Britain, Postgrad Med J 81 (2005) 436-41.
[83] L.A. Santana-Blank, E. Rodriguez-Santana and K.E. Santana-Rodriguez, Photo-infrared pulsed bio-modulation (PIPBM): a novel mechanism for the enhancement of physiologically reparative responses, Photomed Laser Surg 23 (2005) 416-24.
[84] M.L. Kripke, Ultraviolet radiation and immunology: something new under the sun–presidential address, Cancer Res 54 (1994) 6102-5.
[85] P. Whittaker, Laser acupuncture: past, present, and future, Lasers Med Sci 19 (2004) 69-80.
Message from the Doctor
Welcome to “Doctor's Talk,” where I’ll talk about everything related to hair loss and hair transplants. This series is here to help you understand more about how hair treatments work and what you can expect from them. The information provided is based on my 18 years of experience in dealing with hair loss.
Disclaimer -
Please note that this series is purely educational. Reading these posts does not guarantee my services, nor are they intended for business promotion. Information provided is not guaranteed to be up-to-date and should not be considered a substitute for professional medical advice.
Any opinions discussed may not be universally accepted or applicable to all individuals. Always consult a healthcare professional before making any decisions related to your health.

